Սթիվենսի-Ջոնսոնի համախտանիշ և թունավոր վերնամաշկային նեկրոլիզ. դեպքերի շարք և գրականության համառոտ ակնարկ
Տաթևիկ Ալոյան 1, Զարուհի Կալիկյան 2, Սոնա Հարությունյան 2, Ալեքսանդրա Զաքարյան 2, Մարիամ Մովսիսյան 2, Լուսնթագ Կծոյան 2, Օվանես Կարսլյան 3, Արմինե Հակոբյան 1,2
1 Ալերգոլոգիայի և իմունոլոգիայի կլինիկա, «Հերացի» թիվ 1 համալսարանական հիվանդանոցային համալիր, Երևան, Հայաստան
2 Ալերգոլոգիայի և կլինիկական իմունոլոգիայի ամբիոն, Երևանի Մ.Հերացու անվան պետական բժշկական համալսարան, Երևան, Հայաստան
3 Վնասվածքաբանության և օրթոպեդիկ վիրաբուժության ամբիոն, ՀՀ ԱՆ Ակադ Ս.Ավդալբեկյանի անվան առողջապահության ազգային ինստիտուտ, Երևան, Հայաստան
ԱՄՓՈՓԱԳԻՐ
Սթիվենսի-Ջոնսոնի համախտանիշը (ՍՋՀ) և թունավոր վերնամաշկային նեկրոլիզը (ԹՎՆ) դանդաղ տիպի դեղորայքային գերզգայունության հազվագյուտ ձևեր են, որոնք արտահայտվում են մաշկի լայնածավալ մեռուկացմամբ և շերտազատմամբ: Դեղորայքը այս համախտանիշների հիմնական պատճառն է ինչպես մեծահասակների, այնպես էլ երեխաների մոտ: Ամենատարածված պատճառային դեղամիջոցներն են ալոպուրինոլը, արոմատիկ հակաէպիլեպտիկ դեղամիջոցները և սուլֆոնամիդները՝ սեզոնային, աշխարհագրական և էթնիկ տարբերություններով: Այս հոդվածի նպատակն է նկարագրել 2021 թվականի ընթացքում մեր կլինիկայում բարեհաջող վարված ՍՋՀ/ ԹՎՆ կլինիկական դեպքերի շարքը և ներկայացնել հակիրճ գրականության ակնարկ այս թեմայի շուրջ:
Հիմնաբառեր. դեղորայքային ալերգիա, դեղորայքային գերզգայունության ռեակցիաներ, ՍթիվենսիՋոնսոնի համախտանիշ
DOI: 10.54235/27382737-2023.v3.1-67
INTRODUCTION
Drug hypersensitivity reactions (DHR) are Type B reactions that occur when a medication triggers immune or inflammatory cells resulting in unwanted and unpredictable responses. [1,2] Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare forms of delayed-type DHRs with extensive skin necrosis and exfoliation. Historically they have been described as “erythroderma with epidermolysis” by Debré et al. (1939), “unusual bullous eruption” by Lang and Walker in 1956, and finally as “toxic epidermal necrolysis” by the Scottish dermatologist Alan Lyell in 1956, who later declared in his paper “Requiem for Toxic Epidermal Necrolysis” that initially he mistakenly unified in this diagnosis 3 different disease entities that are similar in clinical picture but very distinct in nature. The other 2 diseases were staphylococcal scalded skin syndrome and generalized bullous fixed drug eruption (GBFDE), which present a diagnostic challenge even nowadays. [3,4]
The incidence of SJS and TEN is nearly 0.1-
0.6 and 0.04-0.12 per 100,000, respectively, with a higher prevalence among women (female/ male = 1.7). The overall mortality rate calculated using the SCORTEN system is about 10% and 50%, respectively. [5] Drugs are the main cause of SJS/ TEN in both adults and children. The most common causative medications are allopurinol, aromatic antiepileptics and sulfonamides. Currently, several SJS/TEN predisposing human leukocyte antigen (HLA) haplotypes have been identified for carbamazepine (HLA-B1502 in Han Chinese, HLA-A3101 and HLA-B1511), phenytoin (HLA-B1502) and allopurinol (HLA-B5801).[6]
The most widely accepted pathophysiologic mechanism for SJS/TEN is a pharmacologic interaction with immune receptors, the so-called p-i concept – the unintended action of a drug on T-cell receptors (TCR) by direct binding to them or indirectly by binding to an HLA protein and, thus, presentation to the TCR. This results in T cell activation and subsequent drug inflammatory reactions, such as SJS/TEN, maculopapular eruption/acute generalized exanthematous pustulosis (MPE/AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). [7,8]
The purpose of this paper is to present the cases of SJS/TEN which were successfully treated during 2021 in our department and provide a brief review of the literature.
CASE PRESENTATION
Case 1. A 47-year-old man presented to the emergency department with an acute-onset skin rash and severe burning sensation. The symptoms started gradually as a pruritic rash after 3 hours and during 3 days after taking the first trimethoprim-sulfamethoxazole (TMP/SMX) as a self-treatment of cold pill. He reported that the symptoms progressed despite drug discontinuation and antihistamines. He had no previous allergies until he took TMP/SMX 6 months ago and had a slight rash, which was self-limiting. He has used the same medicine multiple times in the past to self-medicate his cold. The medical history was otherwise unremarkable. On physical examination the skin was covered with extensive areas of erythema and huge bullae, while desquamation with wide areas of erosion as new lesions were appearing at pressure sites (Figure 1). The mucous membranes were not affected. The laboratory test showed leukocytosis (11,000 per mm3) and elevated acute phase proteins with an erythrocyte sedimentation rate (ESR) of 40 mm/h. A diagnosis of SJS/TEN overlap was made. The fluid was aspirated from the most massive bullae to prevent their rupture. The fluid was clear, slightly yellowish, and did not grow microbes when cultured. Along with symptomatic therapy, topical steroids were started on erythematous areas and Boric acid 2% solution and Ethacridine lactate 0.1% dressings on erosions. Steroid pulse therapy was withheld because of the controversial data on its safety and efficacy. Over the next 3 days, an aggravation of the patient’s condition was noted with the appearance of new scaly lesions, and it was decided to start intravenous (IV) infusions of methylprednisolone (Methipred®, ORION Pharma GmbH, Russia)
0.5 mg/kg for 5 days. The patient’s condition slowly improved over the next week with no new lesions at the end of the week. He was discharged on hospitalization day 20 with resolution of erythema and recovering erosions. No skin bacterial superinfections were noted during the inpatient treatment. The patient was advised not to take sulfonamides and had an uneventful 6-month follow up thereafter.
Case 2. A 28-year-old male was admitted to the clinic with a bullous rash on his hands, moderate burning sensation, oral pain and dysphagia. A week ago, he was prescribed oral carbamazepine, risperidone and lorazepam for his mental illness. Mild burning and redness of the palms and oral mucosa appeared 3 days after the first intake of the mentioned medications and progressed over the next 2 days after stopping their use. His mother later recalled him having a similar but milder reaction to carbamazepine twice: a few months ago and a year ago with bullous lesions on the palms only. Upon examination, bullous eruption and erythema were present only on the palms and fingers bilaterally. The oral mucosa was irritated and covered with rounded erosions and white membranous lesions. There were red to black erosions and crusts on the lips (Figure 2). The rest of the skin and mucous membranes were unremarkable. He was afebrile, and laboratory evaluation showed only mild leukocytosis without changes in C-reactive protein (CRP), ESR and basic metabolic panel. SJS or GBFDE was suspected, but biopsy was not possible due to financial issues. Because of concomitant oral mucosal involvement, a diagnosis of SJS was made. Topical mometasone furoate ointment was prescribed along with IV fluids and antihistamines to control the burning sensation. Mouth pain was controlled by mouthwash with 2% lidocaine solution. Over the next few days, the patient’s condition gradually improved. He was discharged 7 days later with healing bullae and erosions and only residual burning sensation in the mouth. At 1-month follow up, the patient was completely symptom-free and strongly avoided carbamazepine thereafter.
Case 3. A 46-year-old male was admitted to the clinic with complaints of a severe rash on the skin of the upper and lower extremities and genital area, intense burning and pain. The first symptoms started 3 days ago in the form of a macular rash with slight burning on the extremities and gradually worsened with the appearance of bullae, mainly on pressure sites. He was transferred to our clinical immunology and allergy clinic from a psychiatric clinic where he had receiving treatment for 40 days with a diagnosis of bipolar disorder with psychotic features. During hospitalization he received several drugs: haloperidol, amitriptyline, lorazepam, diazepam for 40 days and levomepromazine for 10 days. He had been receiving all of these medications multiple times since the diagnosis 15 years ago, except for levomepromazine. He had had 2 similar reactions in the past: a more severe reaction with widespread bullae 1 year ago while taking clozapine (Azaleptine, Arpimed, Armenia), and another 4 years ago while taking an unknown drug. During physical examination, erythema and multiple coalescing bullae up to 5 cm were present on all extremities and in the genital area, covering almost 30% of the body surface area (Figure 3). The oral cavity and conjunctivae were spared, but he mentioned painful urination, presuming an involvement of the urethral mucosa. He had a silver ring that we were unable to remove due to blisters and swelling on the finger. He did not give consent to break the ring. Since the blood supply to the finger was not compromised at that point, we decided to leave the ring in place. The blood workup was within the normal range, except for slight leukocytosis, ESR 30 mm/h and hypoalbuminemia. IV steroids and supportive therapy were started. Despite the methylprednisolone pulse therapy, new lesions were appearing after a week since admission. It was decided to try plasmapheresis, and the patient underwent one session of blood collection (500 mL) and plasmapheresis. His condition remarkably improved the next day. The erythema disappeared, and the bullae and erosions began to dry and heal. He was discharged 4 days later and was asymptomatic at 3 months of follow up.
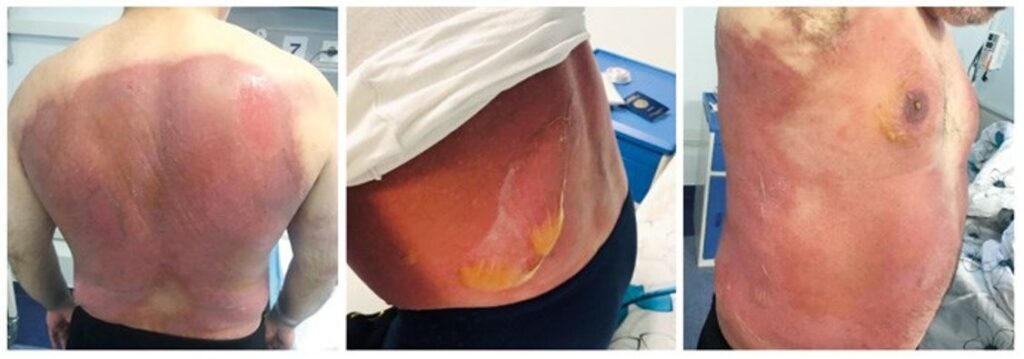
Figure 1. Case 1. Massive bullae and erosions in Stevens-Johnson syndrome and toxic epidermal necrolysis
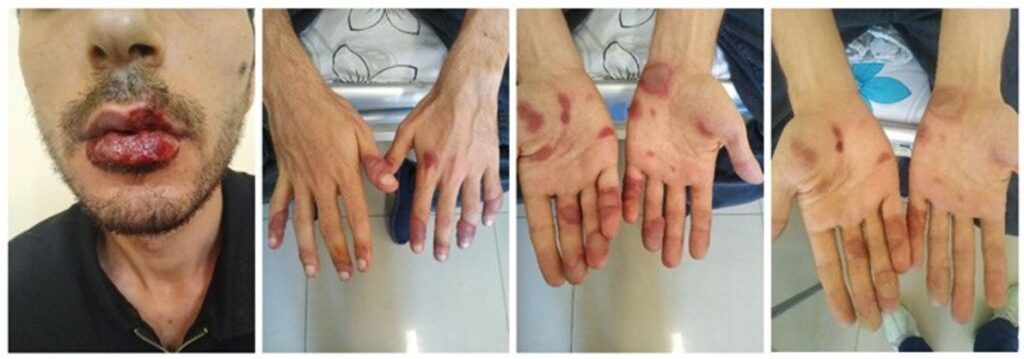
Figure 2. Case 2. Bullous eruptions on the fingers with the involvement of lips and oral mucosa. The first 3 pictures were taken at admission, and the last one at discharge
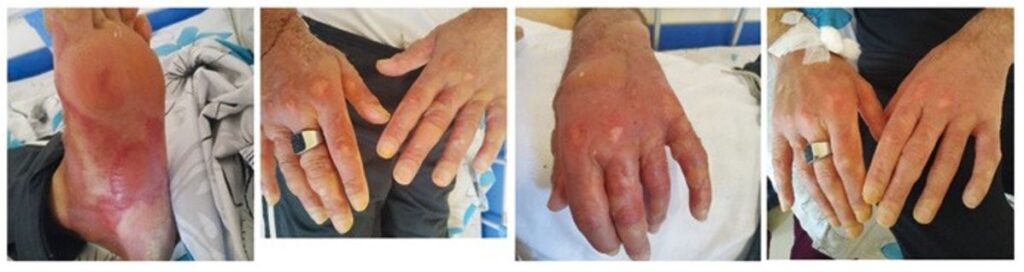
Figure 3. The process of the course of the disease on the patient’s hands
DISCUSSION
Medications are the main cause of SJS/TEN in both adults and children. The most common causative medications are allopurinol, aromatic antiepileptics, and sulfonamides with seasonal, geographical and ethnic variations.[9] In a multicenter study by Micheletti et al. (2018), the most common drug among US patients associated with SJS/TEN was TMP-SMX (89/338; 26.3%) as was our first case.
[10] Meanwhile, in Yang et al. (2018), the most common drug in China, Singapore and Malaysia populations was carbamazepine (29/166, 17.47%; 29/159, 18.24%; and 34/162; 21%, respectively), as in our second case. The second most common drug causing SJS/TEN in these countries was allopurinol. [5]
A Medline and Google Scholar search for TMPSMX-induced SJS/TEN yielded 5 case reports with a mean age of 50.8 years (11-86 years). [11-15] The mean time from the start of medication intake to symptom onset was 5.8 days (1-14 days), starting as urticoid or pruritic maculopapular rash in 2 cases, like in our Case 1, and directly as skin exfoliation in the remaining 3 cases. Systemic steroids were used in 2 cases: methylprednisolone at a dose of 1 g/day was later switched to etanercept in the case reported by Gavigan et al. (2018) and medium-dose steroids in Lipozencic et al. (2002). We started supportive treatment with IV fluids and wound care due to a lack of consensus on the effectiveness of steroids and their potential harm.[16] Later, when the skin exfoliation progressed over the following days and there were no signs of secondary infection, we started IV steroids. The patient’s condition improved over the next few days, but it is uncertain whether this was due to the steroids or the natural course of the disease.
A Medline search for carbamazepine-induced JSJ resulted in 13 case reports with 12 being Asian and only 1 Polish patient. [17-29] Their mean age was 36.85 (12-68) years and 8 were males. The meantime to symptom onset was 10 (2-18) days, with the first symptom being a maculopapular or urticarial rash in 6 patients and directly desquamating bullae in the remaining 7 cases. Mediumor high-dose systemic steroids were used in 10/13 cases, and treatment was not described in 3/13 cases. We decided to manage our case with topical steroids only because of the small body surface affected and only local symptoms.
A search for articles describing levomepromazine (methotrimeprazine)-associated SJS/TEN resulted in only 1 paper (Moubayed et al., 2017) with a case description of 2 Ashkenazi Jewish women (42 and 29 years old) with onset of symptoms 6 and
4 days, respectively, which successfully resolved only after drug discontinuation without any treatment.[30] Both of them later resumed taking the offending drug against physician’s advice with the recurrence of symptoms. The rashes disappeared again after the drug discontinuation. Since this was an extremely rare occurrence for the drug, we are not sure of its causal relationship in our Case 3. However, we did not stop the other drugs he was taking prior to SJS/TEN, with complete resolution of symptoms at discharge.
Thus, the history of drug allergy was simply ignored or forgotten by the 2 patients of our small series, leading to a life-threatening complication. National prescribing and drug control regulations need to be further developed. In addition, a national e-health system that could store all cases of drug allergy would reduce the number of such potentially fatal medical blunders.
CONCLUSION
The diagnosis of delayed-type drug allergy can be difficult due to poor temporal relationships. The clinician should suggest the causality based on the clinical presentation and aspects of the reaction. The development and implementation of a national e-health system that could capture all drug allergy cases for a given patient could prevent at least some of these reactions.
Disclosure Statement
The authors have no conflict of interest to declare.
REFERENCES
- Böhm R, Proksch E, Schwarz T, Cascorbi I. Drug Hypersensitivity. Dtsch Arztebl Int. 2018;115(29-30):501-2.
- Drug hypersensitivity: Classification and clinical features. Available at: https://www.uptodate.com/contents/ drug-hypersensitivity-classification-and-clinical-features?source=autocomplete&index=0~2&search=drug%20hyper. Accessed: July 17, 2022.
- Roujeau, J-C. Epidermal necrolysis (Stevens–Johnson syndrome and toxic epidermal necrolysis): Historical considerations. Dermatologica Sinica. 2013;31.4: 169-74.
- Lyell, A. Requiem for toxic epidermal necrolysis. Br J Dermatol. 1990;122.6: 837-46.
- Yang SC, Hu S, Zhang SZ, et al. The epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in China [published correction appears in J Immunol Res. 2018 Jun 28;2018:4154507]. J Immunol Res. 2018;2018:4320195.
- Yacoub MR, Berti A, Campochiaro C, et al. Drug induced exfoliative dermatitis: state of the art. Clin Mol Allergy. 2016;14(1):9.
- Pichler WJ, Hausmann O. Classification of drug hypersensitivity into allergic, p-i, and pseudo-allergic forms. Int Arch Allergy Immunol. 2016;171(3-4):166-179.
- Pichler WJ. The p-i concept: pharmacological interaction of drugs with immune receptors. World Allergy Organ J. 2008;1(6):96-102.
- Stevens-Johnson syndrome and toxic epidermal necrolysis: Pathogenesis, clinical manifestations, and diagnosis. Available at: https://www.uptodate.com/contents/stevens-johnson-syndrome-and-toxic-epidermal-necrolysis-pathogenesis-clinical-manifestations-and%20diagnosis?search=stevens%20 johnson%20syndrome§ionRank=1&usage_type=default&anchor=H57294570&source=machineLearning&selectedTitle=1~150&display_rank=1#H57294570. Accessed: July 25, 2022.
- Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States [published correction appears in J Invest Dermatol. 2019 Feb;139(2):495-6]. J Invest Dermatol. 2018;138(11):2315-21.
- Rijal JP, Pompa T, Giri S, Bhatt VR. A case of toxic epidermal necrolysis caused by trimethoprim-sulfamethoxazole. BMJ Case Rep. 2014;2014:bcr2013203163.
- Gavigan GM, Kanigsberg ND, Ramien ML. Pediatric Stevens-Johnson syndrome/toxic epidermal necrolysis halted by etanercept. J Cutan Med Surg. 2018;22(5):514-5.
- Langlois MR, Derk F, Belczyk R, Zgonis T. Trimethoprimsulfamethoxazole-induced Stevens-Johnson syndrome: a case report. J Am Podiatr Med Assoc. 2010;100(4):299303.
- See S, Mumford JM. Trimethoprim/sulfamethoxazole-induced toxic epidermal necrolysis. Ann Pharmacother. 2001;35(6):694-7.
- Lipozencic J, Milavec-Puretic V, Kotrulja L, et al. Toxic epidermal necrolysis due to cotrimoxazole. J Eur Acad Dermatol Venereol. 2002;16(2):182-183.
- Stevens-Johnson syndrome and toxic epidermal necrolysis: Management, prognosis, and long-term sequelae. Available at: https://www.uptodate.com/contents/ stevens-johnson-syndrome-and-toxic-epidermal-necrolysis-management-prognosis-and-long-term-sequelae?search=sjs%20treatment&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Accessed: July 27, 2022.
- Avinash A, Amberkar VM, Kunder SK, et al. Carbamazepine-induced life-threatening Stevens-Johnson syndrome and agranulocytosis: the maiden case. J Clin Diagn Res. 2016;10(12):FD01-03.
- Bae HM, Park YJ, Kim YH, Moon DE. Stevens-Johnson syndrome induced by carbamazepine treatment in a patient who previously had carbamazepine induced pruritus a case report. Korean J Pain. 2013;26(1):80-3.
- Capule F, Tragulpiankit P, Mahasirimongkol S, et al. Carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis overlap in a Filipino with positive HLA-B75 serotype. BMJ Case Rep. 2018;2018:bcr2018225028.
- Chowta NK, Chowta MN, Ramapuram J, et al. Carbamzepine-induced toxic epidermal necrolysis. Indian J Crit Care Med. 2011;15(2):123-5.
- Huang LY, Liao WC, Chiou CC, et al. Fatal toxic epidermal necrolysis induced by carbamazepine treatment in a patient who previously had carbamazepine-induced Stevens-Johnson syndrome. J Formos Med Assoc. 2007;106(12):1032-7.
- Jaruthamsophon K, Tipmanee V, Sangiemchoey A, et al. HLA-B*15:21 and carbamazepine-induced Stevens-Johnson syndrome: pooled-data and in silico analysis. Sci Rep. 2017;7:45553.
- Khoo AB, Ali FR, Yiu ZZ, Ferguson JE. Carbamazepine induced Stevens-Johnson syndrome. BMJ Case Rep. 2016;2016:bcr2016214926.
- Nasir SA, Tan HL, Tan HJ, et al. Stevens-Johnson syndrome following failure of genetic screening prior to carbamazepine prescription. Case Rep Dent. 2017;2017:4201357.
- Chao KC, Lu ML, Shen WW. Use of carbamazepine and lamotrigine in a Taiwanese diabetic patient with bipolar disorder. Psychiatry Clin Neurosci. 2012;66(6):538-539.
- Romańska-Gocka K, Gocki J, Placek W, et al. StevensJohnson syndrome after carbamazepine and SJS/TEN overlap syndrome after amoxicillin: case reports and a review. Arch Med Sci. 2010;6(1):130-4.
- Goh TK, Pang SM, Thirumoorthy T, Goh SG. Acute generalised exanthematous pustulosis and toxic epidermal necrolysis induced by carbamazepine. Singapore Med J. 2008;49(6):507-10.
- Liu JJ, Lu SC, Liu JL, Yang HM. Toxic epidermal necrosis induced by carbamazepine embedded in the subcutis. An Bras Dermatol. 2018;93(4):620-1.
- Tenea D. Carbamazepine-induced Stevens-Johnson syndrome sparing the skin previously affected by herpes zoster infection in a patient with systemic lupus erythematosus: a reverse isotopic phenomenon. Case Rep Dermatol. 2010;2(2):140-5.
- Moubayed D, Gifuni AJ, Tourian L. Methotrimeprazineassociated Stevens-Johnson syndrome in 2 Ashkenazi Jewish patients. J Clin Psychopharmacol. 2017;37(1):112-3.