INTRODUCTION
The deterioration of corneal clarity ranks fourth among all causes of blindness in the world, behind age-related macular degeneration, glaucoma, and cataract. Due to various corneal diseases, an estimated 10 million people are bilaterally blind. Even though keratoplasty is still the most common transplant procedure worldwide, demand still outpaces supply. Unfortunately, more than half of the world’s population now lacks access to corneal transplantation. It is necessary to maintain global efforts to promote eye banking in order to combat this, but it is also crucial to create alternative options. [1]
Localized corneal defects that require emergency management, such as microperforations secondary to underlying diseases, remain a challenge to the ophthalmic surgeon. The treatment options for these cases include management of the area of corneal thinning with tissue adhesives, conjunctival flaps, amniotic membrane grafting, patching with scleral lamellae, patching with fresh corneal tissue, or employing glycerin-preserved corneal tissues. [2]
Gamma-irradiated sterile cornea (GISC) is a non-immunogenic, cross-linked, sterile patch graft in which the donor keratocytes and endothelial cells are eliminated. [3,4] The physical and biological characteristics of the GISC are comparable to those of the fresh cornea despite structural changes brought on by irradiation. Gamma-irradiated tissue is a practical and clinically desired choice in a variety of ocular treatments due to these aspects, as well as a lower risk of rejection and a longer shelf life. [5]. This can act as a transplant to cover drainage devices for glaucoma, and can also serve as a tissue graft for repairing severe or impending corneal perforations in tectonic keratoplasty. Considering the restricted availability of fresh corneal tissue in less developed nations, GISC may meet a need for corneal tissue worldwide. Due to the action of irradiation on corneal tissue, it can be employed in circumstances when the corneal endothelium is not required and with a far lower risk of infection and rejection. [6] Grafts that are inappropriate for penetrating keratoplasty but have clean and undamaged stroma are the tissues chosen for gamma irradiation processing. [4]
For complete sterility, GISC is cryogenically and chemically treated, stabilized in medium, and then gamma-irradiated. The GISC falls within the HCT/P category (human cells, tissues, and cellular and tissue-based products). Following the norms and guidelines established by the Eye Bank Association of America, the tissue is first obtained from medically certified donors. The tissues with clear stroma that are unsuitable for penetrating keratoplasty are chosen for gamma irradiation and frozen for preservation and storage. The tissues are taken out of the freezer and placed in a storage solution containing human serum albumin for the sterilizing procedure. According to the American National Standard Sterilization of Health Care Items, this sealed container is sterilized using a verified gamma irradiation method to a sterility assurance level of 10-6. These tissues can be kept at room temperature in human serum albumin for up to 2 years. [1]
By devitalizing corneal cells, this procedure lowers the likelihood of infection transmission and lowers the burden of alloantigens. The shelf-stability of GISC at room temperature for up to 2 years and its transparency with excellent tensile strength are further benefits. GISC is now offered in various forms and sizes with full- or partial-thickness stroma for ocular surgical application. [7]
A study of available material reveals that the physical and biological features of the sterile cornea remain unaffected. Significantly, light transmission qualities of this tissue have been found similar to fresh corneas across all wavelengths. As a result, sterile donor lenticels should behave similarly to fresh corneal tissue when utilized for lamellar corneal treatments that do not need a viable endothelium. Moreover, gamma irradiation lowers tissue allogenicity and may boost resistance to keratolysis by collagenases while decreasing the risk of allogeneic sensitization. These characteristics are especially beneficial in individuals with autoimmune diseases or if repeat keratoplasty is likely in the future (i.e., emergency therapeutic keratoplasty prior to optical keratoplasty). Consequently, GISC tissue might be employed for optical reasons in individuals with viable corneal endothelium, or for therapeutic/tectonic purposes to temporize the full-thickness corneal disease. [1]
Prior to usage in human eyes, the VisionGraft® (Corneagen) sterile cornea tissue was evaluated in vitro. [2] It has been noted to be easy to use, remains transparent in packing without increasing in thickness, and has high tensile strength after suturing. Postoperative results were likewise positive, with excellent biological integration and long-term clarity. [8]
This study aims to present our experience in VisionGraft® surgery in patients with severe ulcers already with microperforations.
METHODS
In 2021, eight surgeries with the use of VisionGraft® GISC have been successfully performed in our clinic.
All 8 patients were referred with corneal ulcers unresponsive to medical treatment. In all cases, PCR for viruses (HSV-1, HSV-2, and VZV) and cultures for bacteria and fungi were performed. The topical and oral treatment was initiated for a week. Due to the lack of response and already existing microperforations, it was decided to immediately proceed with VisionGraft surgery to save the eyeball.
Before the surgery, all 8 patients received a detailed explanation about the advantages and disadvantages of the surgery prior to the procedure, as well as the use of gamma-irradiated tissue for corneal transplantation.
Surgical procedure: All the surgeries were performed by the senior author (A.H.). Patients were anesthetized with a local tetracaine 1% eye drops and retrobulbar injection of lidocaine 2% (3.0 mL). Paracentesis was performed and the anterior chamber was filled with viscoelastic. Trephination was performed with a 12 mm diameter biopsy punch and completed with corneal scissors. Donor grafts were prepared by using 12 mm punches. The donor grafts were sutured to the host bed with 16 interrupted sutures (10.0 nylon).
RESULTS
The clinical photos of the patients before and after the surgeries are shown in Figures 1 to 5.
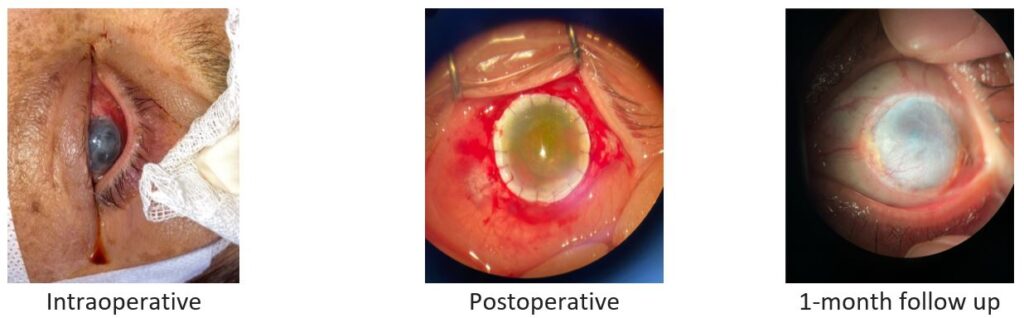
Fig. 1. Patient 1: corneal ulcer caused by herpes simplex virus (HSV-1 and -2) and Staphylococcus epidermidis.
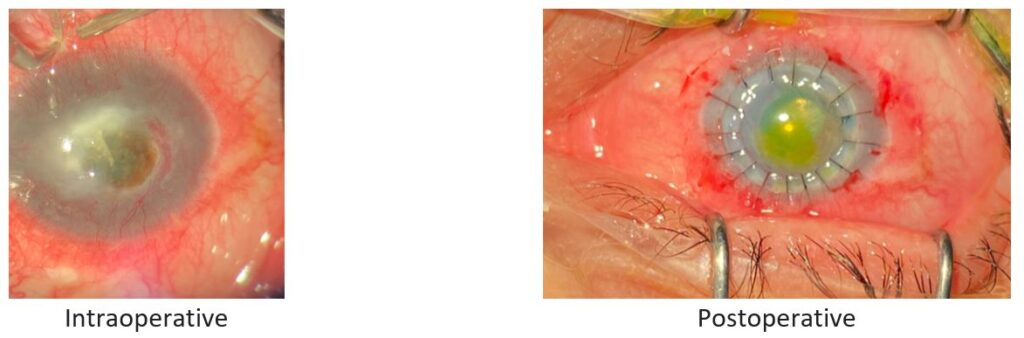
Fig. 2. Patient 2: Stromal necrotizing herpetic keratitis caused by HSV-1.
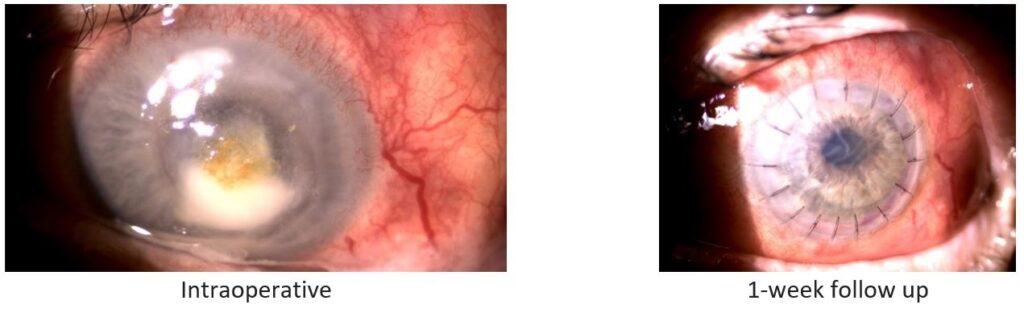
Fig. 3. Patient 3: Bacterial ulcer caused by Streptococcus pneumoniae.
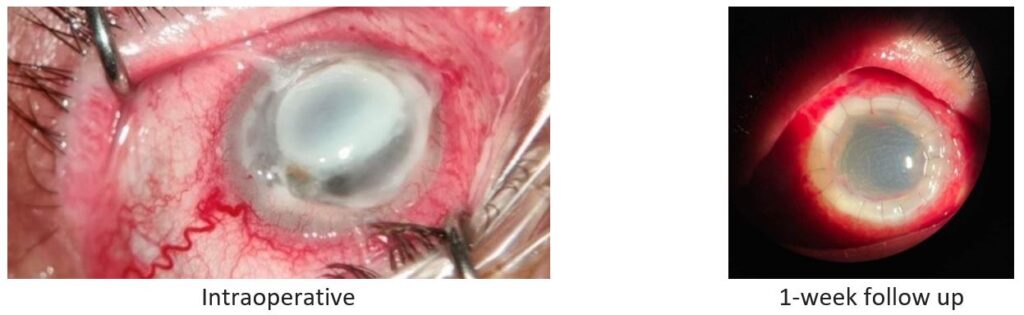
Fig. 4. Patient 4: Stromal necrotizing herpetic keratitis.
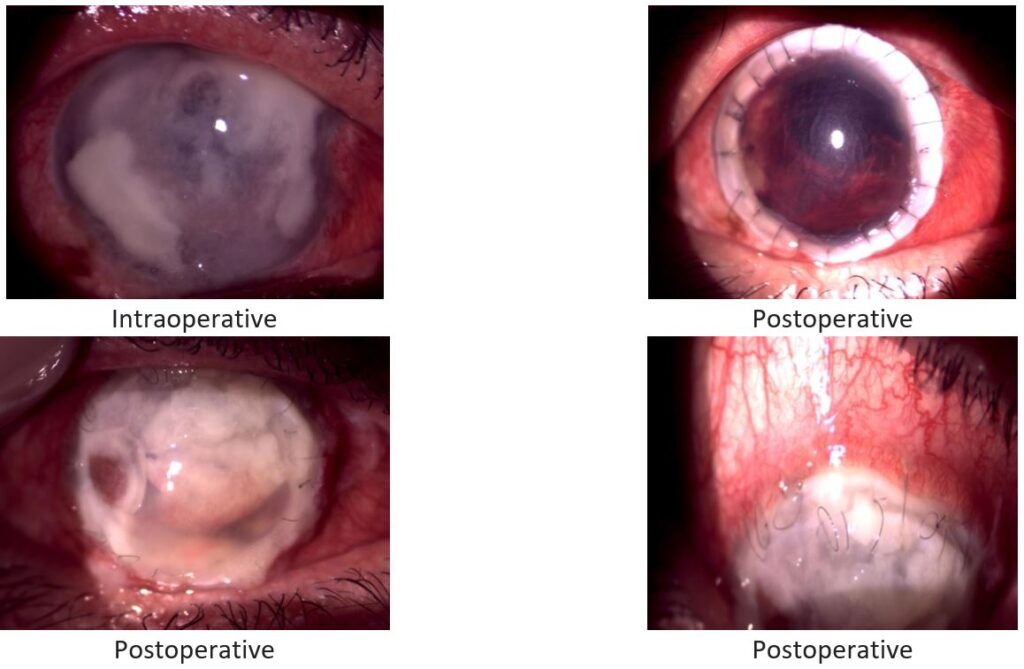
Fig. 5. Patient 5: Herpetic necrotizing stromal keratitis.
In six of the eight patients, the patch graft was effective in restoring tectonic support, which allowed for potential optical keratoplasty at a later time if the decision was made to improve their visual acuity, typically after a year.
Two of the grafts did not epithelialize after surgery and developed sterile corneal melt (both between postoperative months 1 and 3).
All the grafts got opacified within 1-2 months. The infection was eliminated in 6 cases, but in 2 it progressed, and the eyes ended up with development of corneal melt. These 2 patients had a history of systemic hepatitis B virus (HBV) infection.
DISCUSSION
This case series for the first time in Armenia demonstrates the feasibility of using VisionGraft® GISC as a graft tissue for therapeutic intervention to restore the anatomy of the eyeball in the setting of corneal perforation.
Gamma-irradiated corneal tissue, a decellularized stromal collagen matrix, is terminally sterilized and may be stored at room temperature for 2 years. Many investigations have shown that among transplants without functional endothelium, gamma-irradiated corneas remained cleaner than cryopreserved and fresh grafts lacking endothelium. The gamma-irradiated corneas remained thinner than the other two groups, but the difference was not statistically significant. [1] Light microscopy, immunohistochemistry, and electron microscopy investigations of the VisionGraft® revealed intriguing findings, many of which pave the door for more study and work in this field. The ingrowth of keratocytes into the graft’s perimeter was an encouraging observation. These keratocytes are most likely of host origin, which may be validated in future research by HLA typing them and comparing them to host and donor haplotypes. Peripheral epithelial proliferation was also present, but it was very little. The histopathologic discovery of a paucity of axonal regrowth to give neurotrophic support may help to explain this. It is unclear if the VisionGraft® can assist axonal regeneration or whether the lack of such fibers in our case was simply due to a lack of regeneration time and host variables, such our patient’s diabetes mellitus. Clinically, some early epithelial regeneration was seen, although it was at best flimsy [8].
The success of surgery with these corneas at 2-year follow up was established. GISC generally remained intact and appeared well tolerated during this study except 2 cases of corneal melt. We believe that those failed cases were due to the fact that these patients had HBV infection.
Although gamma-irradiated tissue may be a better option to temporize the eye in the setting of emergency for a tectonic procedure and preserve vision potential for future surgery, there is currently а significant shortage of donor tissues in Armenia, which is the single most limiting factor.
The major limitation of this study is the small sample size and relatively short follow-up period.
CONCLUSION
To the best of our knowledge, this is the first case series evaluating the clinical outcomes of using VisionGraft® GISC in Armenia. The availability of tissues is a step towards a cure for corneal blindness worldwide, especially in high-demand areas such as our country.
REFERENCES
- Mathews PM, Fogla R, Samayoa E, et al. Long-term clinical outcomes of keratoplasty using gamma-irradiated corneal lenticules. BMJ Open Ophthalmol. 2019;4(1):e000396.
- Utine CA, Tzu JH, Akpek EK. Lamellar keratoplasty using gamma-irradiated corneal lenticules. Am J Ophthalmol. 2011;151(1):170-4.e1.
- Daoud YJ, Smith R, Smith T, et al. The intraoperative impression and postoperative outcomes of gamma-irradiated corneas in corneal and glaucoma patch surgery. Cornea. 2011;30(12):1387-91.
- Yoshida J, Heflin T, Zambrano A, et al. Gamma-irradiated sterile cornea for use in corneal transplants in a rabbit model. Middle East Afr J Ophthalmol. 2015;22(3):346-51.
- Chae JJ, Choi JS, Lee JD, et al. Physical and biological characterization of the gamma-irradiated human cornea. Cornea. 2015;34(10):1287-94.
- Kuo IC. Review of gamma-irradiated sterile cornea: properties, indications, and new directions. Eye Contact Lens. 2021;47(4):157-62.
- Pan Q, Jampel HD, Ramulu P, et al. Clinical outcomes of gamma-irradiated sterile cornea in aqueous drainage device surgery: a multicenter retrospective study. Eye (Lond). 2017;31(3):430-6.
- Corrales G, Sabet S, Kallakury B, et al. Therapeutic penetrating keratoplasty using full-thickness gamma irradiated corneal tissue. 20 April 2021, PREPRINT (Version 1). Available at: Research Square [https://doi.org/10.21203/rs.3.rs-443990/v1]. Accessed: March 19, 2023.