Нерсес Карамян1,2, Ваге Тер-Минасян2
1Медицинский центр «ИРА Медикал Груп», Ереван, Армения
2Кафедра онкологии, Национальный Институт Здравоохранения МЗ РА им. С. Авдалбекяна, Ереван, Армения
АБСТРАКТ
Радиотерапия играет существенную роль, как компонент комплексного лечения рака молочной железы, практически при всех его стадиях. Однако, несмотря на многочисленные международные рекомендации и клинические протоколы, до сих пор не существует единого подхода среди направляющих на радиотерапию специалистов-онкологов (хирургов и медицинских онкологов). Согласно нашим подсчетам в Армении, по разным причинам, не получает показанную радиотерапию более 100 первичных больных ежегодно. Основной причиной возникновения подобной ситуации является отсутствие утвержденных национальных клинических протоколов, обязательных для всех сертифицированных онкологических подразделений в стране. В таких случаях важную роль должны играть различные профессиональные ассоциации или небольшие инициативные группы, призванные обеспечить единые подходы к лечению на национальном уровне. Удобным форматом для этого является создание небольших рабочих групп, состоящих из ведущих специалистов в конкретной области, которые могут достигнуть консенсуса в изучаемом вопросе.
В связи с этим, 26 ноября 2021 года была проведена рабочая встреча экспертов в области маммологии и радиотерапии, целью которой являлась выработка общих принципов и единого подхода при назначении радиотерапии после проведенной операции и лекарственной терапии при раке молочной железы. В данной публикации приводятся основные выводы, достигнутые в виде консенсуса в ходе обсуждения. Предполагается, что упомянутые выводы послужат в качестве обязательных рекомендаций для всех медицинских центров Армении, занимающихся лечением рака молочной железы.
Ключевые слова: Адъювантная радиотерапия, рак молочной железы, показания, консенсус.
DOI:10.54235/27382737-2022.v2.1-29
PURPOSE OF THE CONSENSUS
To develop common approaches in indication of postoperative irradiation of breast cancer (BC) between leading surgeons in the field of mammology and reconstructive plastic surgery of the breast and radiation oncologists (RO). Eliminate existing discrepancies in the appointment of adjuvant radiotherapy (RT) in Armenia.
INTRODUCTION
Over the past two decades, significant progress has been achieved in the treatment of BC in the world, thanks to advances in the field of diagnostics, radiological imaging and molecular genetics, the introduction of programs for early diagnosis, identification of risk groups, as well as in the field of surgical, radiation and drug treatment methods.
Nevertheless, the problem of BC treatment continues to be one of the most pressing in oncology, due to its prevalence and a permanent increase of morbidity throughout the world. According to the latest published data from the International Center for Research on Cancer, Armenia is no exception. In 2018, 1054 primary cases of BC were detected in Armenia, which is 11.9% of the total number of primary cancers among both sexes in Armenia (Globocan 2018) [1].
As is known, the treatment of BC requires a multimodal approach. Among all the methods used, RT has long occupied its significant and well-defined place as one of the components of complex treatment. There are many publications (forming the basis for the creation of treatment protocols and guidelines) that clearly articulate the role of RT in the treatment of BC. According to a meta-analysis published in 2014, indications for the use of RT are present in approximately 87% of cases of primary BC, which is an “indicator of the optimal utilization of RT” [2].
In Armenia, in 2020, adjuvant RT was performed on 540 BC patients (in the Radiotherapy department of the National Oncology Center and the Radiotherapy Center of “IRA Medical Group”), which is approximately 50% of the total number of primary patients with BC per year. Even if we consider the indicator of the “optimal” use of RT to be somewhat overestimated, and also the fact that a small part of patients could receive treatment outside of Armenia, then, nevertheless, calculations show that about 150-200 patients with BC (i.e., about 15-20%) do not receive the RT indicated to them annually. Another important factor is the lack of a unified approach among breast surgeons and medical oncologists to refer patients for RT, i.e. there is an individual interpretation of well-known guidelines, depending on specific cases.
CONSENSUS
The main reason for this situation is the lack of approved national clinical protocols that are mandatory for all certified oncology units in the country. In such cases, various professional associations or small initiative groups should play an important role in order to ensure uniform approaches to treatment at the national level. A convenient format for this is to set up small working groups, composed of leading experts in a particular field, who can reach a consensus on the issue under study.
On November 26, 2021, at the initiative of the Radiotherapy Center “IRA Medical Group”, which has been operating in Armenia since 2019, a working meeting of experts in the field of mammology and RT was held aiming to develop common principles and a unified approach when prescribing RT after surgery and drug therapy for BC.
The following experts (all from Yerevan – the capital city of Armenia) were invited to the meeting:
► Leading breast surgeons
► Asilbekyan G. (“Astghik” Medical Center)
► Avetisyan A. (National Oncology Center)
► Berberyan N. (“Erebouni” Medical Center)
► Kocharyan A. (Armenian-American Wellness Center)
► Prof. Sahakyan A. (“Artmed” Medical Center)
► Stepanyan A. (“Nairi” Medical Center)
► Leading radiation oncologists
► Arustamyan M. (“IRA Medical Group” Medical Center)
► Prof. Karamyan N. (“IRA Medical Group” Medical Center)
► Lazaryan A. (National Oncology Center)
► Muradyan L. (National Oncology Center)
► Saghatelyan T. (National Oncology Center).
As a result of the meeting, the “Armenian consensus on indications for adjuvant RT after surgical and drug treatment (COBRA)” was reached.
During the meeting, three main issues were presented for discussion:
- Indications for RT after organ-preserving operations (COBRA-1)
- Indications for RT after radical mastectomy (COBRA-2)
- Indications for RT after reconstructive plastic surgery (COBRA-3).
RESULTS
INDICATIONS FOR RT AFTER ORGANPRESERVING OPERATIONS (COBRA-1)
Since the 2000s, various organ-preserving operations (sectoral resections, quadrantectomy, lumpectomy, etc.) have become widespread, replacing radical mastectomy (ME) in most cases. This approach was based on the results of a number of large published studies that demonstrated that Breast Conserving Treatment (BCT) has been proven to be equivalent to ME with regards to overall survival, and in the past few years, large retrospective series have implied that BCT was even superior to ME in T1-2N0-2 breast cancer [3].
Performing such organ-preserving operations requires mandatory use of adjuvant RT, both in cases of ductal cancer in situ (DCIS) and in invasive BC of various stages (see National Comprehensive Cancer Network [NCCN] Guidelines Version 2.2022 DCIS-1, BINV-2, BINV-14) [4].
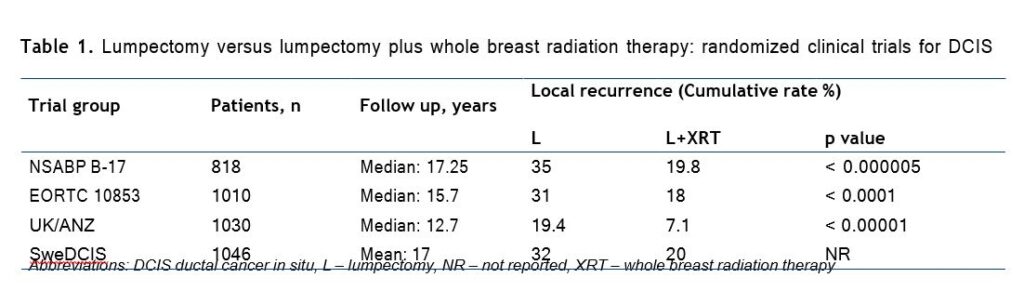
Four prospective randomized controlled trials (RCT) of excision only versus excision plus breast irradiation for DCIS have been performed with reported results, and all have shown that the rate of local recurrence (LR) was reduced with the addition of radiation (Table 1) [5].
A meta-analysis was completed utilizing the individual patient data from each of the four trials men tioned above. With a total of 3729 women eligible for analysis, it was demonstrated that RT reduced the absolute 10-year risk of any ipsilateral breast event by 15.2%. This analysis further established strong and consistent evidence that the addition of RT following breast-conserving surgery for DCIS reduced the risk of LR by approximately 50% [6].
In a multivariate analysis, performed within the NSABP study, factors associated with an increased risk of LR were: < 40 years of age, clinically symptomatic presentation (nipple discharge or palpable mass), intermediate or poor differentiation, solid/comedoand cribri-form histology, involved or uncertain margins and treatment by local excision alone [7]. Despite this, several recent studies have attempted to identify and treat patients with highly selected favorable tumor characteristics with excision alone (i.e., without whole-breast irradiation) and report 10-year local failure rates of 3% to 25%. One of these studies has proposed a scoring system using histopathologic features including tumor size, grade, and margin width in an attempt to stratify patients according to local failure risk after excision plus or minus whole-breast irradiation. Each variable was assigned a score of 1 to 3, and the sum total defined the Van Nuys Prognostic Index. Although appealingly simple, this scheme is drawn from the retrospective analysis of a patient cohort which has several methodologic shortcomings, and it has not been independently validated [6]. After discussing the presented data, the working initiative group made a number of recommendations on the indications for RT after organ-preserving operations for BC: Consensus on the indications of RT after organ-preserving operations in breast cancer (COBRA-1): ► DCIS TisN0M0 High Risk Patients – palpable mass, larger size, higher grade, close margins, age < 50 (risk of recurrence about 50%) ► Invasive BC cT1-3 cN0 (no need for patients T1N0 with age > 70, ER+, who receive adjuvant endocrine therapy)
► Invasive BC cT1-3 cN+
► After neoadjuvant CHT if cN+ and if inoperable initially.
INDICATIONS FOR RT AFTER RADICAL MASTECTOMY (COBRA-2)
Despite the trend towards organ-sparing operations designed to ensure quality of life and cosmetic/functional effect, the number of radical ME performed continues to be high, which is explained by the high percentage of cases of locally advanced BC. Patients who present with locally advanced BC require care from a multidisciplinary team that incorporates diagnostic imaging, chemotherapy, surgery, and careful pathology assessment, including molecular-based studies, radiation, and, if indicated, biologic and hormonal therapies. Fortunately, the outcome for patients with locally advanced BC has improved dramatically. Before the routine use of chemotherapy, patients treated with ME, radiation, or a combination of the two had high rates of distant metastases and death. The introduction of adjuvant and neoadjuvant chemotherapy and hormone therapy regimens has significantly improved the prognosis [5].
However, despite chemotherapy, total breast removal with axillary lymph node dissection for locally advanced BC, adjuvant RT is often necessary, depending on the stage of the disease, the status of the lymph nodes, risk factors, etc. In order to ensure local control in locally advanced BC, a clear understanding of the role of RT after ME is necessary. According to the latest NCCN guidelines (see BINV-14 and BINV-3), RT is not indicated after ME for tumors < 5 cm, negative lymph nodes, and clear resection margins > 1 mm. RT is strongly recommended for tumors > 5 cm, questionable or positive resection margins, positive lymph nodes (Category 1). When prescribing RT, risk factors must also be taken into account. RT is also performed even in cases where a pathological complete response (pCR) of the tumor is achieved after preoperative chemotherapy, but there was a clinical stage of cN+.
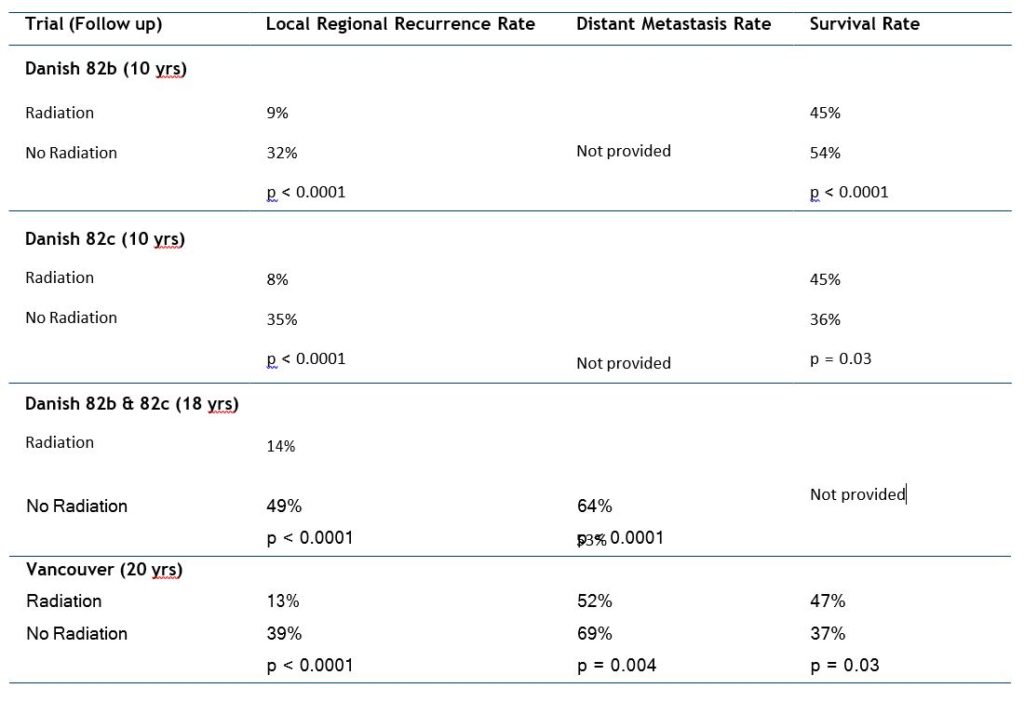
The three most recently completed RCTs investigating the efficacy of post-mastectomy RT for patients with stage II or III BC were conducted in the 1980s and have 15to 20-year outcome data. The largest of these studies was the Danish Breast Cancer Cooperative Group 82b trial, which randomly assigned 1708 premenopausal women with stage II or III BC to receive ME followed by 9 cycles of chemotherapy or ME, RT, and 8 cycles of CMF [Cyclophosphamide-Methotrexate-Fluorouracil] chemotherapy. At the same time, this group also conducted the 82c trial, in which > 1300 postmenopausal women were randomly assigned to undergo ME and 1 year of tamoxifen or ME, tamoxifen, and RT. Finally, a smaller trial, conducted in Vancouver, Canada, randomly assigned 318 premenopausal women with lymph node-positive disease to undergo ME and CMF chemotherapy with or without post-ME RT [8].
The results of these studies demonstrating the effectiveness of adjuvant RT after chemotherapy and ME are summarized in Table 2.
Several important concepts can be ascertained from these studies. First, these studies clearly demonstrated that by reducing local-regional recurrence, post-mastectomy radiation therapy (PMRT) could improve overall survival. Second, these trials demonstrated that these patients had a clinically relevant risk of local-regional recurrence despite the use of either chemotherapy or tamoxifen. These findings imply that the benefits of systemic treatments are predominantly to lower the competing risk of distant metastases, which makes the achievement of local-regional control more important [8, 9].
Another published study investigating this issue compared the outcomes of 579 patients who received neoadjuvant chemotherapy, ME, and RT with those of 136 patients who were treated with neoadjuvant chemotherapy and ME. Patients in this study had been treated in prospective chemotherapy trials in which RT was given on the basis of physician recommendations and patient preferences. Therefore, the patients with worse disease characteristics were more often treated with RT. Despite this, the local-regional recurrence rate was found to be significantly lower in the group treated with PMRT than in the group treated with neoadjuvant chemotherapy and ME (10-year local-regional recurrence rates were 8% and 22%, respectively; p = 0.001). For patients with clinical stage III disease or extensive disease after chemotherapy, RT led to significant improvements in local-regional recurrence and overall and cause-specific survival rates.
The same group of investigators also showed that among patients with stage III disease who achieved a pCR, the local-regional recurrence rate for those treated with RT was 7% versus 33% for those who did not receive RT (p = 0.040) [10, 11].
Table 2. Local regional recurrence, rates of distant metastasis and overall survival in randomized trials comparing the use of post-mastectomy radiation for patients treated with mastectomy and systemic therapy [7]
In summary, the use of PMRT is reasonable for all patients with clinical T3 or T4 tumors or clinical stage III disease regardless of their response to the chemotherapy regimen. In terms of clinical stage I or II breast cancer, PMRT should be recommended for patients with 4 or more positive lymph nodes after chemotherapy and for the unusual patient in whom the disease progresses and the primary tumor exceeds 5 cm in diameter.
Based on the above arguments, we put forward a number of recommendations as a consensus on the use of RT after ME.
Consensus on indications for RT after mastectomy in locally advanced breast cancer (COBRA 2):
► No RT if negative lymph nodes, tumor < 5.0 cm, margins > 1 mm
► RT for pT2 with close margins (consider high risk recurrence factors: central/medial tumors, > 2 cm with < 10 lymph nodes removed, grade 3, ER-negative, LVI-positive, young age )
► RT for cT3-4 and for any T with cN+ or pN+
► Positive margins (if re-resection not feasible).
INDICATIONS FOR RT AFTER RECONSTRUCTIVE PLASTIC SURGERY (COBRA-3)
Breast conserving treatment (BCT) has been proven to be equivalent to ME with regards to overall survival, and in the past few years, large retrospective series have implied that BCT was even superior to ME in T1-2N0-2 BC. Nevertheless, the rate of ME is increasing especially in the United States, mostly as a result of performing MEs with immediate breast reconstruction (IBR) in patients with early-stage BC and bilateral MEs for unilateral disease. Parallel to that, there is an increase in the rate of patients who are referred to PMRT, mainly following the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) publication in 2014. The increasing rates of PMRT are also observed in the setting of IBR, which may represent increasing experience and confidence with irradiating the chest wall after breast reconstruction, regardless of the type of procedure.
Coordination of radiation and breast reconstruction is a commonly encountered issue for patients treated with ME and requires clear communication between surgical oncologist, reconstructive/plastic surgeon, radiation oncologist, and the patient. There are many factors to consider regarding the issue of reconstruction and PMRT, including ensuring the safety and efficacy of radiation treatments, ensuring the maximal quality of life for the patients, and achieving the optimal long-term aesthetic result from the procedure [12, 13].
The two major classes of reconstruction are implant-based approaches and autologous tissue reconstruction. The two options for timing for the reconstruction are immediate – done at the time of ME – or delayed – done after completion of RT. There are advantages and disadvantages of both approaches and both timings. Implant-based approaches are simpler surgical procedures that avoid the donor-site morbidities of autologous tissue transfers. In addition, implants can be used in thin women who do not have adequate volume of autologous tissue in donor sites. Typically, for this procedure, a tissue expander is placed under the pectoralis major muscle and, after full expansion is achieved, replaced with an implant. Most women treated with PMRT who undergo implant-based reconstruction require an immediate reconstruction procedure. This is because after RT the normal tissues are less compliant, and tissue expanders are often unsuccessful and may cause rib fractures and other injuries. For women treated with autologous tissues, the reconstruction can be immediate or delayed. Immediate reconstruction has the benefit of being accompanied by a skin-sparing ME, which preserves a significant component of the normal breast skin and preserves the natural inframammary sulcus and other skin envelopes. These elements are important to achieving the optimal cosmetic outcome. The downsides of immediate reconstruction relative to delayed reconstruction are twofold: radiation has adverse effects on the long-term aesthetics of breast reconstructions, particularly implant-based reconstruction, and reconstruction has a negative effect on the design and delivery of radiation treatment fields [5].
Based on current evidence that risk-reducing ME in women at high risk for BC (e.g., BRCA carriers) reduces the risk of subsequent BC by 85-95%, and that breast volume-reducing surgery in standard-risk females reduces the risk of subsequent BC by approximately 28%, a key assumption is that any residual breast glandular tissue (rBGT) poses a “risk” for recurrence or subsequent new BC in these patients. The absolute risk is dependent on the patient’s individual risk to develop BC. These include patient tumorand treatment-related factors, such as age, genetics, amount of rBGT, risk for recurrent disease (e.g., nodal status, lymphovascular invasion, tumor biology), extent of surgery, RT, and systemic therapy [14, 15].
Importantly, approximately 5-10% of the glandu lar tissue is retained after conventional total ME. It is essential to include rBGT within the irradiation volumes. The recommendations for it are based on the observation that most of the LRs after ME occur at the level of the skin and subcutaneous tissue (range, 72-100%), where most of the rBGT and draining lymphatics are found. The second most common site of recurrence is within the pectoral muscle, especially near the primary tumor site (028%) [14, 15].
The results of a systematic review of PubMed publications performed to document the spatial location of LR after ME were published in 2020. A total of 3922 titles were identified, of which 21 publications were eligible for inclusion in the final analysis. A total of 6901 ME patients were included (range, 25-1694). The mean LR proportion was 3.5%. Among the total of 351 LR lesions, 81.8% were in the subcutaneous tissue and the skin, while 16% were pectoral muscle recurrences [16].
The aesthetic change of the breast, as a consequence of treatments, develops in the majority of patients who undergo an immediate reconstruction and PMRT. In general, implant-based reconstruction has high rates of late contraction, fibrosis, implant fixation, and poor aesthetic outcome. Many of these changes begin 6 months after treatment and insidiously progress over time. Different authors report the wide range of complications after IBR and PMRT (from 27% to 50%) [4]. At the same time, the rate of complications after autologous tissue reconstructions is considerably lower. Although some studies have suggested that PMRT in the setting of reconstruction increases the relative rate of complications regardless of the type (implant or autologous) and the timing of reconstruction, fewer complications and better long-term cosmetic outcome have been reported when an autologous flap-based reconstruction was performed compared to IBR in combination with PMRT. The IBR has 2.64 times higher odds of complications (95% CI 1.77, 3.94, p < 0.001) than autologous-flap-based reconstruction [17, 18]. Current PMRT techniques used in the post-IBR setting are still often field-based rather than volume-based such that the target volume frequently includes the implant or reconstructed breast itself. The use of modern volume-based RT planning may reduce the dose to normal tissue and thereby treatment-related toxicity, without compromising target coverage [17, 18]. Thus, despite the higher percentage of complications during RT after plastic breast reconstruction, the indications for RT remain the same as after conventional ME. Below are the conclusions of the Armenian Consensus on this issue. Indications for RT after reconstructive plastic surgery (COBRA-3): ► No RT if negative lymph nodes, tumor < 5.0 cm, margins > 1 mm
► RT for pT2 with close margins (consider high risk recurrence factors: central/medial tumors, > 2 cm with < 10 lymph nodes removed, grade 3, ER-negative, LVI-positive, young age )
► RT for cT3-4 and for any T with cN+ or pN+
► Positive margins (if re-resection not feasible).
CONCLUSIONS
The main conclusions reached in the form of consensus during the discussion should serve as mandatory recommendations for all medical centers in Armenia involved in the treatment of breast cancer until the national clinical protocols on this issue are approved.
Conflict of interests
The authors have no conflict of interest on the submitted material.
REFERENCES
- Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [published correction appears in CA Cancer J Clin 2020;70(4):313]. CA Cancer J Clin. 2018;68(6):394-424.
- Barton MB, Jacob S, Shafiq J et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144
- van Maaren MC, de Munck L, de Bock GH et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016;17:1158-1170
- NCCN Clinical Practice Guidelines in Oncology; Breast Cancer. Version 2.2022-December 20, 2021
- Halperin EC, Wazer DE, Perez CA, Brady LW. Perez and Brady’s Principles and Practice of Radiation Oncology. 7th edition. USA, Wolters Kluwer, 2019:3939-4417
- Early Breast Cancer Trialists’ Collaborative Group, Correa C, Mc Gale P, Taylor C et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;41:162-167
- Donker M, Litiere GW, Julien J-P et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcomes after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;31:4054-4059
- Danish Breast Cancer Cooperative Group, Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24(15):2268-2275
- Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82(3):247-253
- Kyndi M, Overgaard M, Nielsen HM et al. High local recurrence risk is not associated with large survival reduction after postmastectomy radiotherapy in high-risk breast cancer: a subgroup analysis of DBCG 82 b&c. Radiother Oncol. 2009;90(1):74-79
- Rusthoven CG, Rabinovitch RA, Jones BL et al. The impact of postmastectomy and regional nodal radiation after neoadjuvant chemotherapy for clinically lymph node-positive breast cancer: a National Cancer Database (NCDB) analysis. Ann Oncol. 2016;27(5):818-827
- van Maaren MC, de Munck L, de Bock GH et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016;17:1158-1170
- Doherty C, Pearce S, Baxter N et al. Trends in immediate breast reconstruction and radiation after mastectomy: a population study. Breast J. 2020;26:446-453
- Grinstein O, Krug B, Hellmic M et al. Residual glandular tissue (RGT) in BRCA1/2 germline mutation carriers with unilateral and bilateral prophylactic mastectomies. Surg Oncol. 2019;29:126-133
- Papassotiropoulos B, Guth U, Chiesa F et al. Prospective evaluation of residual breast tissue after skinor nipple-sparing mastectomy: results of the SKINI-Trial. Ann Surg Oncol. 2019;26:1254-1262
- Kaidar-Person O, Poortmans P, Offersen BV et al. Spatial location of local recurrences after mastectomy: a systematic review. Breast Cancer Res Treat. 2020;183(2):263-273
- Kaidar-Person O, Offersen BV, Hol S et al. ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother Oncol. 2019;137:159-166
- Kim SH, Kim JM, Park SH et al. Analysis of the effects of breast reconstruction in breast cancer patients receiving radiotherapy after mastectomy. Arch Plast Surg. 2012;39(3):222-226